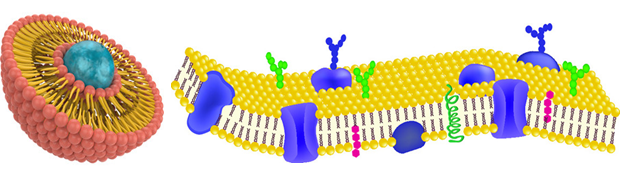
A phospholipid has the unique property of amphiphilic. It has both lipophilic (water-loving) and hydrophobic (water-fearing) properties. These dual traits of phospholipids spontaneously form a bilayer when put in the water, but how does that happen?
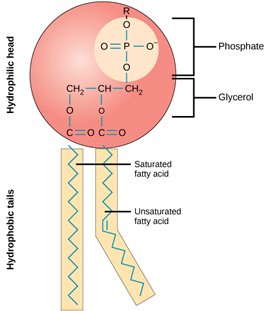
A phospholipid consists of one glycerol, two fatty acids, and an inorganic phosphate group modified by an alcohol. They bonded through a chemical reaction of carboxylic acid and alcohol, called esterification. The fatty acids are attached to two carbons of the glycerol molecule, while the phosphate group modified by alcohol is attached to the third carbon of the glycerol backbone.
Phospholipids have two sections. The phosphate group stays at the head section, while fatty acids make up the tail section. The Head of the phospholipid is hydrophilic (water-loving) which attracts water molecules and binds readily through hydrogen bonds. The fatty acids side which makes up the tail are hydrophobic (water-fearing), making it insoluble to water. Phospholipids form a bilayer in water where the hydrophilic heads of the molecules are exposed to the water, while the hydrophobic tails bury themselves and aggregate together in the middle.
Contents
Contents
What is the phospholipid bilayer made of?
The amphipathic properties of water urge the phospholipid bilayer formation. There are two adjacent sheets of phospholipids forming a bilayer. The phosphate head is directly in contact with water creating a barricade to avoid fatty acid tails from contacting the water. The fatty-acid chains meet at the center to stay away from the water.
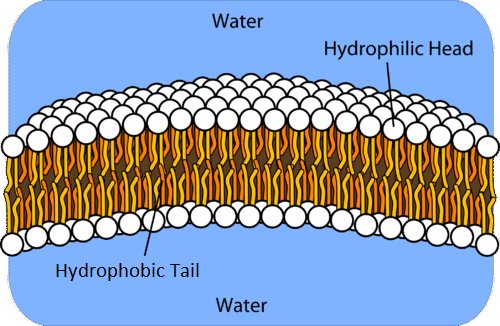
The phosphate is negatively charged, making it polar and hydrophilic (water-loving). Thus, the phosphate heads tend to attract water molecules through hydrogen bonding. In contrast, the phospholipid tails are uncharged, non-polar, and hydrophobic. Hence, it repels water.
How do phospholipids behave in water?
Phospholipids do not mix well with water because of their amphipathic properties (characterized by having both hydrophilic and hydrophobic traits). Having both properties is driven by the structural component of phospholipid: the fatty acid tail and the phosphate group head. One is hydrophilic, and the other is hydrophobic. This unique property affects its behavior with water molecules. But how does it interact with water?
Phospholipids have different formations depending on the amount of phospholipids concentration in water. If you pour a few drops of phospholipids in the water, they float to the surface and form an oily film. The hydrophilic polar heads turn toward the water, while the hydrophobic nonpolar tails aggregate inside and interact with each other.
As more phospholipids saturate the water, few will start to form in a spherical aqueous molecule, called micelles, as a response to the amphipathic nature of phospholipids. The hydrophilic heads turn toward the water, while the hydrophobic tails aggregate inside and interact with each other.
As phospholipids continue to saturate water, they spontaneously form into a bilayer. Two adjacent polar layers will directly contact water molecules creating a barricade, while the fatty acid ends bury themselves in the middle. More phospholipids drenched in the water would naturally form spherical bilayers of phospholipids, which are similar to cell membranes and organelles.
Is phospholipid soluble in water?
Water is known as the universal solvent. It is correct on many occasions because of its polar arrangement. Water has two partially positive charged hydrogen ends and one partly negative charged oxygen end, making water a polar-covalent that attracts other polar molecules. Although water can dissolve more substances than any other liquid, it cannot dissolve non-polar molecules.
In the case of phospholipids’ amphipathic property, water cannot dissolve phospholipids totally because the hydrocarbon tails of two fatty acids are hydrophobic. The Fatty acid chains are non-polar and never interact with water and tend to move away. On the other hand, the phosphate group end is hydrophilic and readily dissolves in water. (https://winandoffice.com/)
What part of phospholipid is hydrophobic and hydrophilic?
Phospholipid structure consists of one glycerol, two fatty acids, and a phosphate group modified by alcohol compounds. The phosphate group is polar (i.e., it has an electric charge and is attracted to water). The long fatty-acid chains are non-polar (i.e., it is uncharged and repels water). Therefore, the phosphate group is hydrophilic, and the fatty-acid tails are hydrophobic.
What’s the importance of phospholipid’s insolubility to water ?
Phospholipids are abundant in all life forms, mostly found on cell membranes and organelles. The behavior of phospholipids towards water has a great sense on the function of cell membranes. To be more specific, its amphipathic (water-loving and water-fearing) nature caused the formation of a lipid bilayer, a critical component of cell membranes.
The phospholipid is partially insolubility caused by the hydrophilic fatty acids that urges the lipid bilayer formation. They act as a barrier and protect the cells and organelles. It also permits selective passage of particular substances into and out of cells. Moreover, it facilitates the transportation and absorption of essential fats (e.g., omega-3 fats) throughout the bloodstream.